You are now leaving this site
This link will take you to a third-party site that is not owned by Adaptimmune. Click “OK” if you would like to proceed or click “Cancel” to stay on this site.
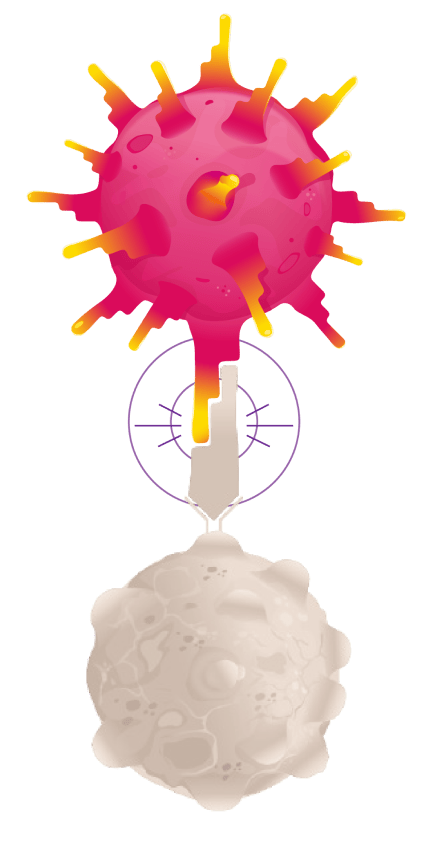
TECELRA is a different type of treatment than what you may have previously received—cells are taken from your body, engineered, and placed back into your body to fight synovial sarcoma cells.
 T CELL
T CELL
 T-CELL RECEPTOR (TCR)
T-CELL RECEPTOR (TCR)


T cells play a big role in your body's immune response. They have special proteins on their surface, known as T-cell receptors (TCRs), that can recognize things that may harm the body like tumor cells. This allows them to target and destroy the dangerous cells.

T cells play a big role in your body's immune response. They have special proteins on their surface, known as T-cell receptors (TCRs), that can recognize things that may harm the body like tumor cells. This allows them to target and destroy the dangerous cells.
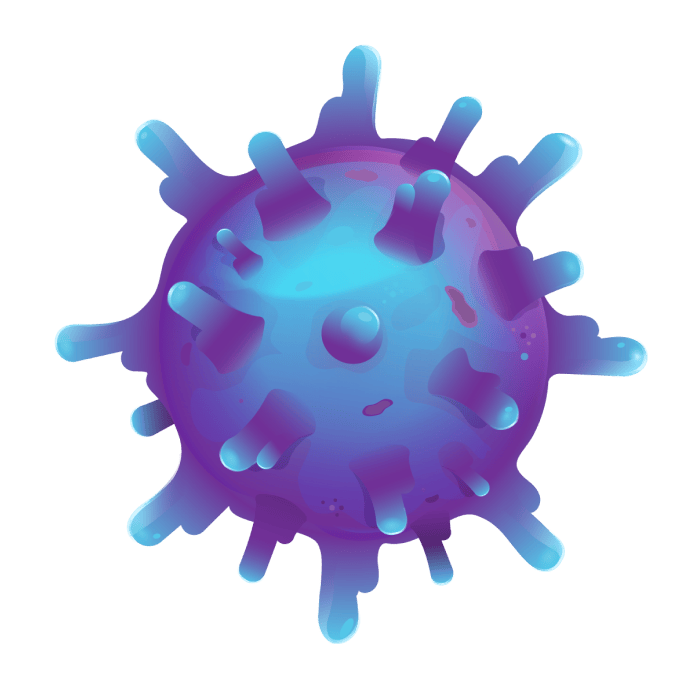
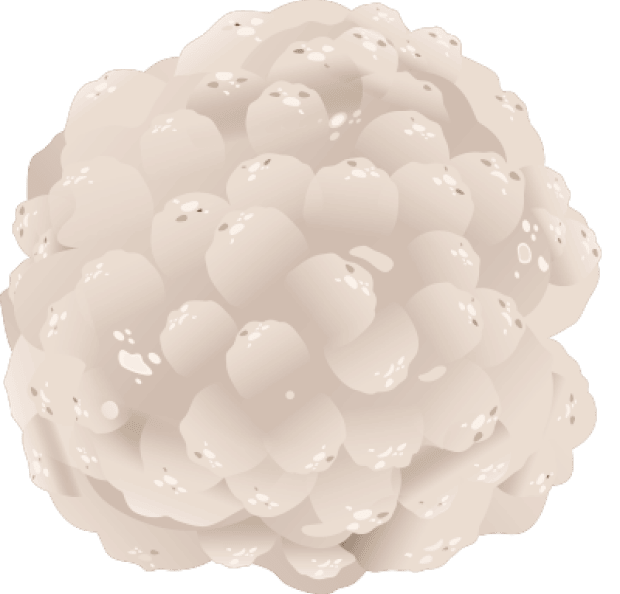 SYNOVIAL SARCOMA TUMOR
SYNOVIAL SARCOMA TUMOR
 SYNOVIAL SARCOMA CELL
SYNOVIAL SARCOMA CELL


Synovial sarcoma cells, like other cancer cells, try to hide from your T cells by stopping or limiting the display of certain proteins on their surface. These proteins are how T cells identify cancer cells, so this makes it harder for T cells to find and attack the cells.


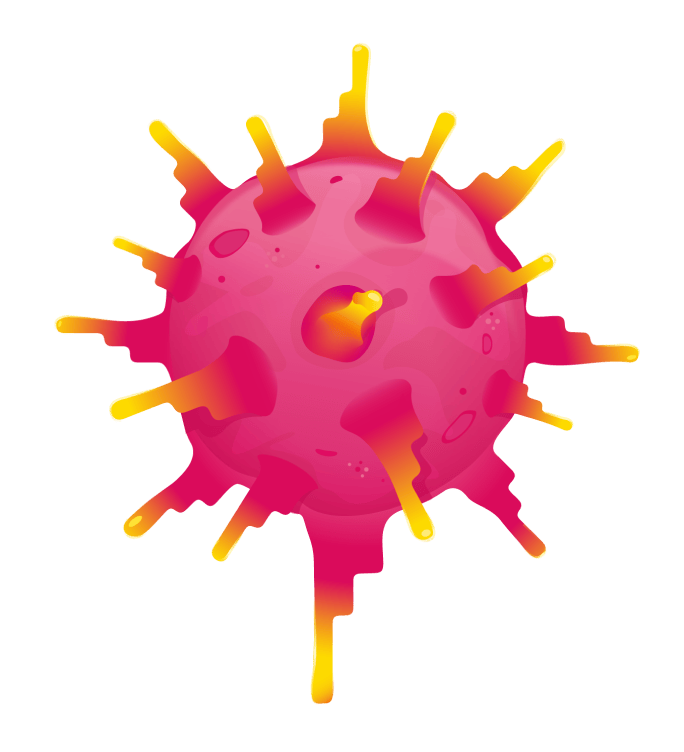
 ENGINEERED T CELL
ENGINEERED T CELL

 ENGINEERED TCR
ENGINEERED TCR


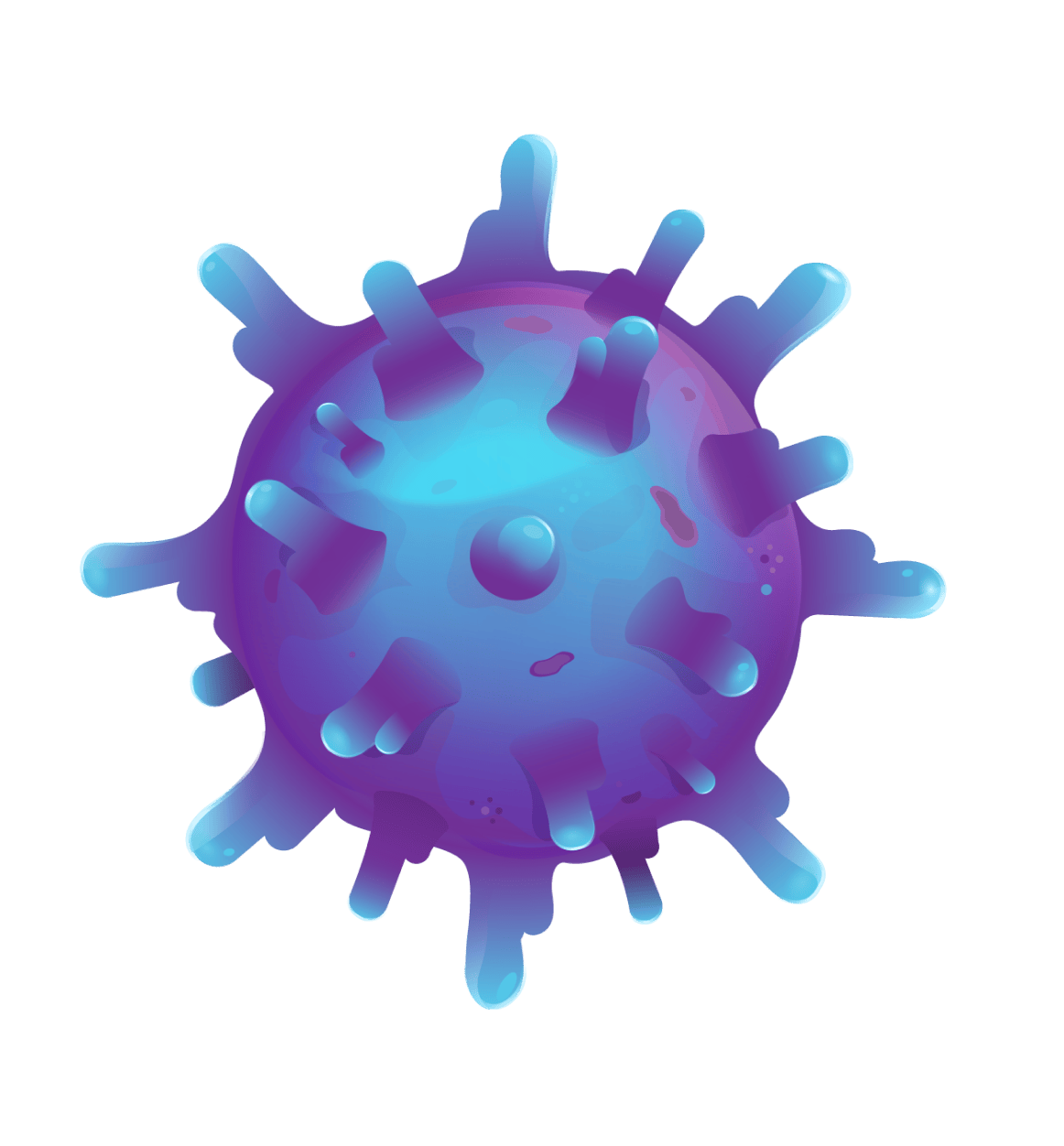
TECELRA is made by collecting your own T cells and engineering them to have modified TCRs. These TCRs have an enhanced ability to recognize a type of protein called MAGE-A4 that can be displayed by synovial sarcoma cells.

Synovial sarcoma cells, like other cancer cells, try to hide from your T cells by stopping or limiting the display of certain proteins on their surface. These proteins are how T cells identify cancer cells, so this makes it harder for T cells to find and attack the cells.



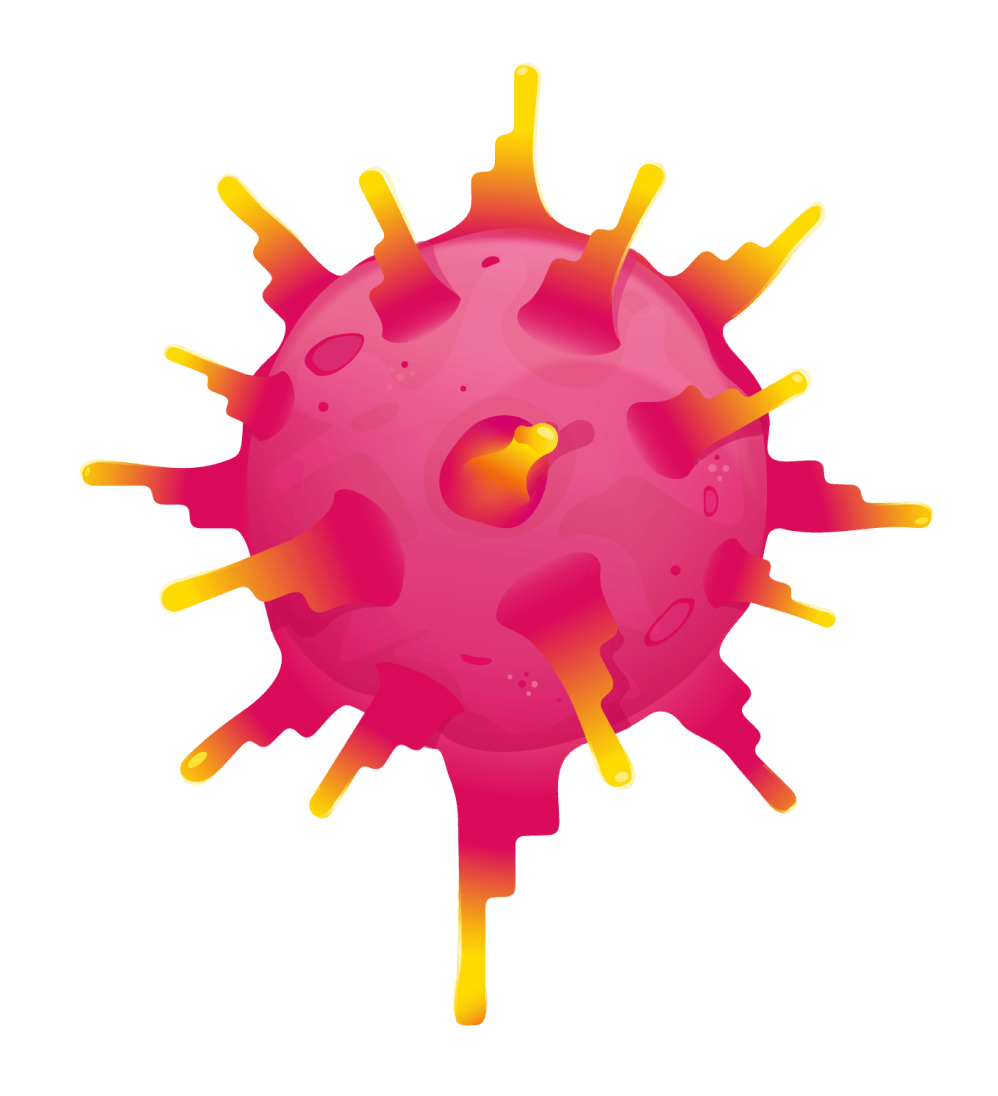
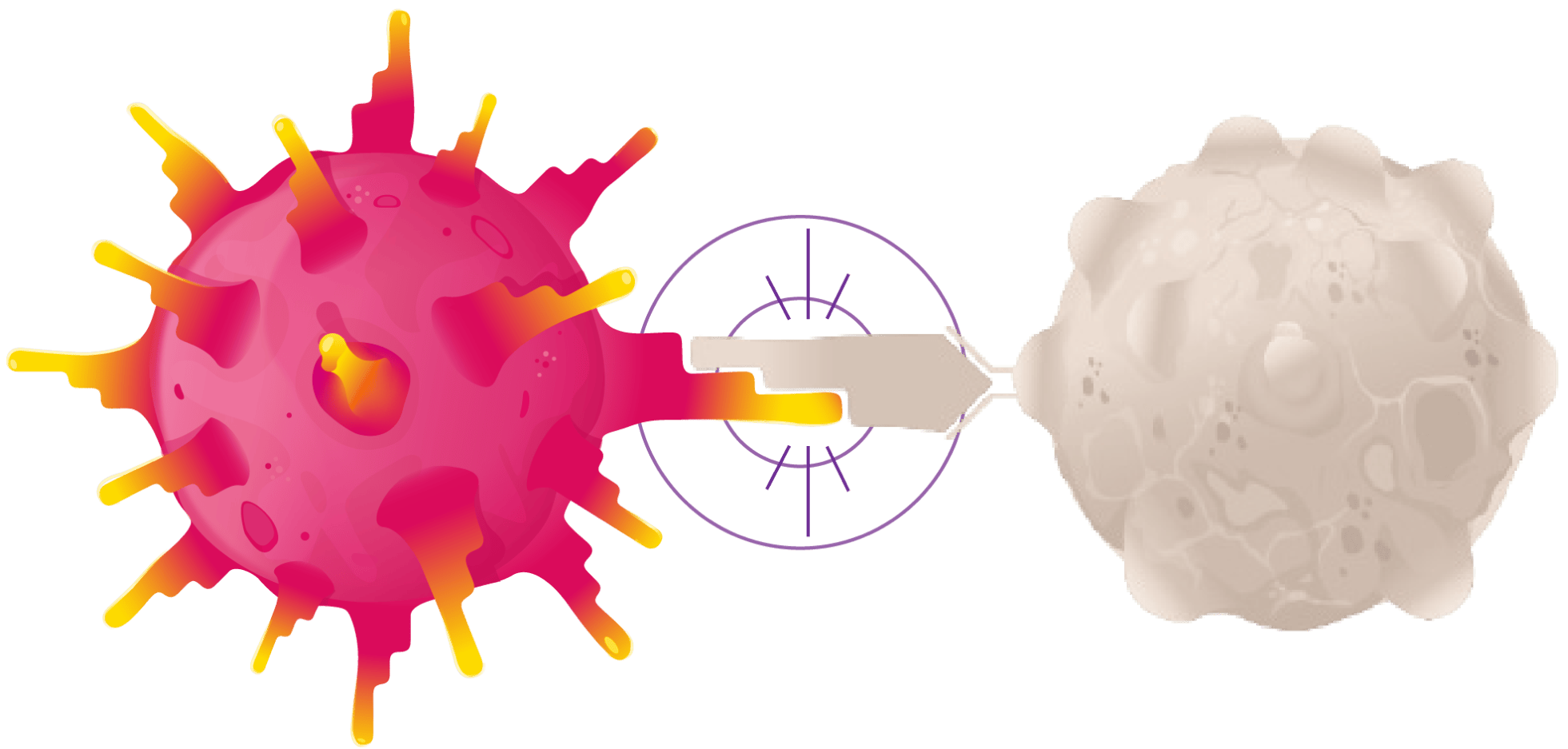 ENGINEERED T CELL
ENGINEERED T CELL
 ENGINEERED TCR
ENGINEERED TCR

 SYNOVIAL SARCOMA CELL
SYNOVIAL SARCOMA CELL


Once engineered, the T cells with enhanced TCRs are put back into your body in a single infusion, where they target and destroy synovial sarcoma cells.


TECELRA is a medicine, called a genetically modified autologous T cell immunotherapy, that is used to treat synovial sarcoma. It is used when other kinds of treatment do not work. TECELRA is different from other cancer medicines because it is made from your own white blood cells that are made to recognize and attack your cancer cells. Your healthcare provider will perform tests to see if TECELRA is right for you. TECELRA is approved based on patient response data. Additional data are needed to confirm the clinical benefit of TECELRA. It is not known if TECELRA is safe and effective in children.
Please see Medication Guide, including Important Warning.
Important Warning: You will likely be in a hospital before and after getting TECELRA. TECELRA may cause side effects that can be severe or life-threatening. Call your healthcare provider or get emergency help right away if you get any of the following: fever (100.4°F/38°C or higher); chills/shivering; difficulty breathing; fast or irregular heartbeat; low blood pressure; fatigue; severe nausea, vomiting, or diarrhea; severe headache; or new skin rash. Tell all your healthcare providers that you were treated with TECELRA.
After getting TECELRA, you will be monitored daily at the healthcare facility for at least 7 days after the infusion. You should plan to stay close to a healthcare facility for at least 4 weeks. Do not drive, operate heavy machinery, or do other activities that could be dangerous for at least 4 weeks after you get TECELRA. Your healthcare provider will do blood tests to follow your progress. It is important that you have your blood tested. If you miss a scheduled appointment for your collection of blood, call your healthcare provider as soon as possible to reschedule.
Before you receive TECELRA, tell your healthcare provider about all the medicines and supplements you take and your medical conditions, including: seizure, stroke, confusion, or memory loss; heart, liver, or kidney problems; low blood pressure; lung or breathing problems; recent or active infection; past infections that can be reactivated following treatment with TECELRA; low blood counts; pregnancy, you think you may be pregnant, or plan to become pregnant; breastfeeding; or taking a blood thinner.
The most common side effects of TECELRA include nausea, vomiting, fatigue, infection, constipation, fever (100.4°F/38°C or higher), abdominal pain, difficulty breathing, decreased appetite, diarrhea, low blood pressure, back pain, fast heart rate, chest pain, general body swelling, low white blood cells, low red blood cells, and low platelets.
You are encouraged to report side effects to the FDA at (800) FDA‑1088 or www.fda.gov/
Please see Medication Guide, including Important Warning.
TECELRA is a medicine, called a genetically modified autologous T cell immunotherapy, that is used to treat synovial sarcoma. It is used when other kinds of treatment do not work. TECELRA is different from other cancer medicines because it is made from your own white blood cells that are made to recognize and attack your cancer cells. Your healthcare provider will perform tests to see if TECELRA is right for you. TECELRA is approved based on patient response data. Additional data are needed to confirm the clinical benefit of TECELRA. It is not known if TECELRA is safe and effective in children.
Please see Medication Guide, including Important Warning.
Important Warning: You will likely be in a hospital before and after getting TECELRA. TECELRA may cause side effects that can be severe or life-threatening. Call your healthcare provider or get emergency help right away if you get any of the following: fever (100.4°F/38°C or higher); chills/shivering; difficulty breathing; fast or irregular heartbeat; low blood pressure; fatigue; severe nausea, vomiting, or diarrhea; severe headache; or new skin rash. Tell all your healthcare providers that you were treated with TECELRA.
After getting TECELRA, you will be monitored daily at the healthcare facility for at least 7 days after the infusion. You should plan to stay close to a healthcare facility for at least 4 weeks. Do not drive, operate heavy machinery, or do other activities that could be dangerous for at least 4 weeks after you get TECELRA. Your healthcare provider will do blood tests to follow your progress. It is important that you have your blood tested. If you miss a scheduled appointment for your collection of blood, call your healthcare provider as soon as possible to reschedule.
Before you receive TECELRA, tell your healthcare provider about all the medicines and supplements you take and your medical conditions, including: seizure, stroke, confusion, or memory loss; heart, liver, or kidney problems; low blood pressure; lung or breathing problems; recent or active infection; past infections that can be reactivated following treatment with TECELRA; low blood counts; pregnancy, you think you may be pregnant, or plan to become pregnant; breastfeeding; or taking a blood thinner.
The most common side effects of TECELRA include nausea, vomiting, fatigue, infection, constipation, fever (100.4°F/38°C or higher), abdominal pain, difficulty breathing, decreased appetite, diarrhea, low blood pressure, back pain, fast heart rate, chest pain, general body swelling, low white blood cells, low red blood cells, and low platelets.
You are encouraged to report side effects to the FDA at (800) FDA‑1088 or www.fda.gov/
Please see Medication Guide, including Important Warning.
This link will take you to a third-party site that is not owned by Adaptimmune. Click “OK” if you would like to proceed or click “Cancel” to stay on this site.